Spiritual Well-being and Quality of Life for Patients Undergoing Chemotherapy
Pancreatic Cancer Blog – Commentary on Articles and AbstractsHere is where we take complex medical articles and break them down into language you and I
For over 22 years pancreatica.org has provided current, credible, and comprehensive information to those in need.
Cancer Patient’s Alliance is a 501(c)(3) non-profit organization. All donations are tax-deductible.
94% of all revenue goes towards our programs, with only 6% towards MANAGEMENT AND GENERAL EXPENSES.
PROPOSED: Every newly diagnosed person with pancreatic cancer (ductal adenocarcinoma of the pancreas) should receive genetic screening prior to beginning treatment – to test for germline genetic mutations in the homologous recombination DNA repair pathway, including genes such as BRCA1, BRCA2, PALB2, and others.
These results, in from 12% to 17% of pancreatic cancer patients, suggest that treatment that includes DNA cross-linking agents such as platinum compounds or PARP inhibitors may be superior to standard best practices therapy.
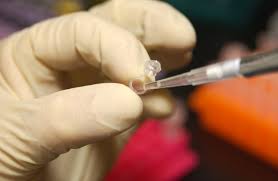
OFFER: Color Genomics offers a 30-gene cancer panel for $224 (normally $249) when the Promotion Code “PANCREATIC” is entered at checkout (price will reduce upon entering this code). This is a physician-ordered saliva kit.
For more information [Click Here]
RATIONALE: The age of precision medicine in pancreatic cancer is approaching but has not yet fully arrived. This new realm will likely come a time in the not-too-distant-future when treatments are exactly tailored to specific individual cancers. Or, perhaps the treatment will be selected as the very best option for a defined subtype of pancreatic cancer per genetic fingerprint indicating likelihood of response.
By way of a small concrete example, one notes the recent instance of the now FDA-approved pembrolizumab (Keytruda) demonstrating an improved treatment response for the treatment of those with prior treated pancreatic cancer who show a positive MSI-H biomarker, a sign of microsatellite instability due to the inability of the cell to repair DNA mismatch mistakes.
One can further see evidence toward this future, for example, in the recent advances in immunotherapy for cancer, such as that of Car-T gene therapy in acute lymphoblastic leukemia.
And largely, the past three years has shown remarkable collaborative research work published by impressive pancreatic cancer research scientists and institutions that has identified many of the genetic mutations associated with pancreatic cancer. This has been accompanied by early efforts to postulate genetic practical subtypes of pancreatic cancer.
We are not completely there yet, but testing can help identify one practical subgroup
The BRCA genes intersect and participate as a part of a greater protein cascade known as the Fanconi Anemia pathway that also include such genes such as ATM, PALB2, RAD 51 and others. Mutations of genes in this sequence are associated with increased pancreatic cancer incidence though dysfunction in DNA repair involving homologous recombination.
For this subgroup, it increasingly appears that ductal adenocarcinoma of the pancreas may respond better to chemotherapy that includes DNA cross-linking agents (e.g., cisplatin, or poly ADP ribose polymerase [PARP] inhibitors, etc.). Apart from other recent research, promising results of a Phase I study including gemcitabine, cisplatin AND a PARP inhibitor under such circumstance in advanced pancreatic cancer were published in January 2018, and a similar phase II clinical trial is currently in process.1,2
It is perhaps important to note that BRCA and other gene status in this subgroup is established by patient and not tumor testing.
These testing results are aimed at identifying those 12% to 17% of patients for whom the addition of a cross-linking drug agent may confer survival advantage.8 It will not change standard best practices therapy for the majority of those with pancreatic cancer.
Thus, it is our modest proposal at this time that every newly diagnosed person with pancreatic cancer receive genetic testing prior to beginning treatment – to test for germline genetic mutations in the homologous recombination DNA repair pathway.
And with treatment consideration to follow testing results. The 30-gene cancer screening test referenced here above is not exhaustive, but perhaps represents a feasible and affordable reasonable current-practices step. More comprehensive genetic testing measures are also viable options.
What To Do:
1). If you have been newly diagnosed with pancreatic cancer, consult your medical team in these matters.
2). For more screening test information [Click Here]
3). If you go this route, use the code “PANCREATIC” at checkout for your discount.

Pancreatic cancer is a serious disease. Taking an aggressive rational stance at the earliest possible time, supported by the best medical team, and treated in the most appropriate manner gives the best chance for survival.
We believe in strong patient-physician bonds, scientifically-based treatment, and that comfort can come from knowing that everything that reasonably can be done – is being done.
That the best approach is meeting cancer of the pancreas head-on and armed with the best available information.
James Abbruzzese, MD Chief, Medical Oncology Duke University
Markus Büchler, MD Chairman, Surgery Heidelberg University, Germany
Ralph Hruban, MD Director, GI / Liver Pathology Johns Hopkins University
Eileen O’Reilly, MD Associate Director for Clinical Research – Memorial Sloan-Kettering Cancer Center
Margaret Tempero, MD Chief, Medical Oncology University of California at San Francisco
BIBLIOGRAPHY
Retrospective Survival Analysis of Patients with Resected Pancreatic Ductal Adenocarcinoma and a Germline BRCA or PALB2 Mutation.Yu S, Agarwal P, Mamtani R, Symecko H, Spielman K, O’Hara M, O’Dwyer P, Schneider C, Teitelbaum U, Nathanson KL, Domchek SM, Reiss KA., JCO Precision Oncology, DOI: 10.1200/PO.18.00271, March 28, 2019. [Click Here]
Maintenance Rucaparib in BRCA1, BRCA2 or PALB2 Mutated Pancreatic Cancer That Has Not Progressed on Platinum-based Therapy. Abramson Cancer Center of the University of Pennsylvania. Clinical trial published at clinicaltrials.gov; Accessed 3/11/2019. [Click Here]
Niraparib in Patients with Pancreatic Cancer. Dana Farber Cancer Institute. Clinical trial published at clinicaltrials.gov; Accessed 3/11/2019. [Click Here]
Phase 1 trial evaluating cisplatin, gemcitabine, and veliparib in 2 patient cohorts: Germline BRCA mutation carriers and wild-type BRCA pancreatic ductal adenocarcinoma. O’Reilly EM, Lee JW, Lowery MA, Capanu M, Stadler ZK, Moore MJ, Dhani N, Kindler HL, Estrella H, Maynard H, Golan T, Segal A, Salo-Mullen EE, Yu KH, Epstein AS, Segal M, Brenner R, Do RK, Chen AP, Tang LH, Kelsen DP., Cancer. 2018 Jan 16. doi: 10.1002/cncr.31218. PMID: 29338080. [Click Here]
Clinical Trial NCT01585805: A Randomized Phase II Study of Gemcitabine, Cisplatin +/- Veliparib in Patients with Pancreas Adenocarcinoma and a Known BRCA/ PALB2 Mutation (Part I) and a Phase II Single Arm Study of Single-Agent Veliparib in Previously Treated Pancreas Adenocarcinoma (Part II). Start Date April 18, 2012; Estimated End Date November 30, 2018. Sponsor: NCI, Principal Investigator: Eileen O’Reilly, Memorial Sloan Kettering Cancer Center. [Click Here]
Defects in homologous recombination repair genes are associated with good prognosis and clinical sensitivity to DNA-damaging agents in pancreatic cancer: A case report. Sonnenblick A, Zick A1, Maoz M, Cohen S, Kadouri L, Peretz T, Hubert A. Mol Clin Oncol. 2018 May;8(5):683-685.
[Click Here]
Association between homologous recombination repair gene mutations and response to oxaliplatin in pancreatic cancer. Kondo T, Kanai M, Kou T, Sakuma T, Mochizuki H, Kamada M, Nakatsui M, Uza N, Kodama Y, Masui T5, Takaori K, Matsumoto S, Miyake H, Okuno Y, Muto M. Oncotarget. 2018 Apr 13;9(28):19817-19825. [Click Here]
Genomics-Driven Precision Medicine for Advanced Pancreatic Cancer: Early Results from the COMPASS Trial. Aung KL, et al. Clin Cancer Res. 2017 Dec 29. doi: 10.1158/1078-0432.CCR-17-2994. [Epub ahead of print] PMID: 29288237. [Click Here]
Homologous Recombination Deficiency and Platinum-Based Therapy Outcomes in Advanced Breast Cancer. Zhao EY, et al., Clin Cancer Res. 2017 Dec 15;23(24):7521-7530. doi: 10.1158/1078-0432.CCR-17-1941. PMID: 29246904. [Click Here]
DNA Repair Dysfunction in Pancreatic Cancer: A Clinically Relevant Subtype for Drug Development. Golan T, Javle M., J Natl Compr Canc Netw. 2017 Aug;15(8):1063-1069. doi: 10.6004/jnccn.2017.0133. PMID: 28784866. [Click Here]
Therapeutic targeting and patient selection for cancers with homologous recombination defects. Talens F, Jalving M, Gietema JA, Van Vugt MA. Expert Opin Drug Discov. 2017 Jun;12(6):565-581. doi: 10.1080/17460441.2017.1322061. PMID: 28425306 [Click Here]
Germline mutations in pancreatic cancer and potential new therapeutic options.
Pihlak R, Valle JW, McNamara MG., Oncotarget. 2017 Apr 20;8(42):73240-73257. doi: 10.18632/oncotarget.17291. PMID: 29069866. [Click Here]
Therapeutic Implications of Molecular Subtyping for Pancreatic Cancer.
Pishvaian MJ, Brody JR., Oncology (Williston Park). 2017 Mar 15;31(3):159-66, 168. PMID: 28299752. [Click Here]
Whole Genome Sequencing Defines the Genetic Heterogeneity of Familial Pancreatic Cancer. Roberts NJ, et al., Cancer Discov. 2016 Feb;6(2):166-75. doi: 10.1158/2159-8290.CD-15-0402. PMID: 26658419 [Click Here]
Genomic analyses identify molecular subtypes of pancreatic cancer.
Bailey P, et. al., Nature. 2016 Mar 3;531(7592):47-52. doi: 10.1038/nature16965. Epub 2016 Feb 24. PMID: 26909576 [Click Here]
Whole genomes redefine the mutational landscape of pancreatic cancer.
Waddell N, et al., Nature. 2015 Feb 26;518(7540):495-501. doi: 10.1038/nature14169. PMID: 25719666. [Click here]
Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation.
Kaufman B, et al., J Clin Oncol. 2015 Jan 20;33(3):244-50. doi: 10.1200/JCO.2014.56.2728. PMID: 25366685 [Click Here]
Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Golan T, Kanji ZS, Epelbaum R, Devaud N, Dagan E, Holter S, Aderka D, Paluch-Shimon S, Kaufman B, Gershoni-Baruch R, Hedley D, Moore MJ, Friedman E, Gallinger S. Br J Cancer. 2014 Sep 9;111(6):1132-8. doi: 10.1038/bjc.2014.418. PMID: 25072261 [Click Here]
An emerging entity: pancreatic adenocarcinoma associated with a known BRCA mutation: clinical descriptors, treatment implications, and future directions.
Lowery MA, Kelsen DP, Stadler ZK, Yu KH, Janjigian YY, Ludwig E, D’Adamo DR, Salo-Mullen E, Robson ME, Allen PJ, Kurtz RC, O’Reilly EM. Oncologist. 2011;16(10):1397-402. doi: 10.1634/theoncologist.2011-0185. PMID: 21934105 [Click Here]
Complete remission, in BRCA2 mutation carrier with metastatic pancreatic adenocarcinoma, treated with cisplatin based therapy.
Sonnenblick A, Kadouri L, Appelbaum L, Peretz T, Sagi M, Goldberg Y, Hubert A. Cancer Biol Ther. 2011 Aug 1;12(3):165-8. PMID: 21613821 [Click Here]

Choose a walk, run, or any other activity that you like and work to raise donations leading up to your Event! Simply set up your participation page and we will be in touch!

Already registered for your next Race? Dedicate your participation to Pancreatic Cancer. Simply set up your participation page and together we will work towards our shared mission!

Creating a Facebook Fundraiser is easy: Click the Link below and Facebook will take you through the steps! Dedicate your Birthday or another important date to honor a loved one.
Join us on Social Media !
Participate in #GivingTuesday for Pancreatic Cancer on December 3rd 2019!!
Click Here to Add a Calendar Reminder!




HONOR A LOVED ONE
BY SHARING YOUR STORY
You can honor your loved one by sharing your story with us. Whether it’s your personal story, honoring a friend or relative’s journey, or why you support the cause, we want to hear from you!
Amazon donates 0.5% of the price of your eligible AmazonSmile purchases to help fight pancreatic cancer!
AmazonSmile is the same Amazon you know. Same products, same prices, same service. Simply use our Link to start shopping!
Pancreatic cancer is the most aggressive of the major cancers, has the highest mortality rate, and is the LEAST funded.
CLICK HERE TO MAKE A TAX-DEDUCTIBLE DONATION !
Call us at: 831-372-0900 or Email: participate@tofightcancer.com
Our mission is to promote awareness, increase education, and further pancreatic cancer research, specifically research aimed at early diagnosis.
Studies show that death rates for pancreatic cancer are increasing while for most other cancers they are declining, we need change !
Early diagnosis is key:
Survival increases dramatically if patients are diagnosed in time for surgery!
For medical information Click Here for our sister site Pancreatica.org
Pancreatica.org is a top-rated internet resource containing unique educational resources for patients, their caregivers, physicians and researchers, that displays an impressive concentration of scientific articles.

Currently, no standard marker exists to diagnose the disease in early stages. Our aim is to support the development of a marker for early diagnosis. If the disease is found even a year earlier, outcomes will begin to improve. LEARN MORE

Currently, only about 15% of those diagnosed with pancreatic cancer are eligible for potentially curative surgery. We need to find the disease earlier!
We are dedicated to promoting awareness, increasing education, and furthering pancreatic cancer research.
Pancreatic Cancer Blog – Commentary on Articles and AbstractsHere is where we take complex medical articles and break them down into language you and I
Pancreatic Cancer Blog – Commentary on Articles and AbstractsHere is where we take complex medical articles and break them down into language you and I
Pancreatic Cancer Blog – Commentary on Articles and AbstractsHere is where we take complex medical articles and break them down into language you and I
This study is basically looking at whether aspirin can make a chemotherapy drug called gemcitabine work better for pancreatic cancer. You’ve probably heard of aspirin; it’s the stuff you take for a headache or fever.
Cancer Patients Alliance is a 501(c)(3) non-profit. Initiatives include, ToFightCancer.com and Pancreatica.org. All Donations are tax-deductible. Pancreatic cancer is the least funded cancer in terms of research.
Despite causing enormous mortality, pancreatic cancer receives (on a mortality basis) much less funding for research than most of the other major cancers. Currently, there is no molecular marker or genetic screening tool to aid in the earlier diagnosis or screening of pancreatic cancer. Treatment results would improve significantly if this cancer could be diagnosed at an earlier stage.
© 2024 Pancreatica. All rights reserved | Privacy Policy
312 Fountain Avenue, Pacific Grove, California 93950 | Phone: (831) 658-0600 | Fax: (831) 658-0518 | participate@tofightcancer.com