Here in the Pancreatica Blog we have reported previous studies documenting patients with pancreatic cancer (ductal adenocarcinoma of the pancreas) with specific genetic characteristics such BRCA1 and BRCA2 mutations who have demonstrated robust treatment responses to DNA crosslinking agent “chemotherapy” such as platinum salts, PARP [poly ADP-ribose polymerase enzyme] inhibitors, and others. By way of shorthand for the purposes of this blog entry, shall we call these pancreatic cancer patients Platinum Responders?
Mounting evidence appears to suggest that the group of pancreatic cancer Platinum Responders may be LARGER than previously understood, as the BRCA genes and subsequent protein cascading proteins that they control appear to “intersect” with another protein cascade known as the Fanconi Anemia pathway that can be traced back to a number of genetic mutations including in such genes such as ATM, FANCC, FANCG, PALB2, and perhaps others also associated with increased pancreatic cancer incidence. Of course, this is the cutting edge of medicine and science, and the results are coming from disparate medical studies, so we need to be careful in these assertions at the present time. But should more comprehensive study bear out these early results, the value in terms of possible duration of survival and treatment response for a subset of patients with pancreatic cancer, cannot be overstated. The key, should this prove true, is to distinguish those with pancreatic cancer who would benefit from genetic testing, identifying this subset of patients in order to match the right treatment to the right patients.
Today’s paper under discussion by Holter et al. (link at the end of this blog entry, and included as an element in the chart immediately below), measures the occurrence of pancreatic cancer patients who carry inherited germline BRCA1 or BRCA2 mutations, and recommends guidelines for genetic testing in this circumstance. Unlike for breast or ovarian cancers, there are no USPSTF prevention guidelines for genetic testing of pancreatic cancer patients. A positive BRCA discovery may provide an opportunity for more effective treatment. Other patients that could possibly benefit in terms of the selection options for pancreatic cancer treatment may demonstrate acquired somatic BRCA1 or BRCA2 mutations (or those in select genes related to the Fanconi pathway) related to their tumors, but germline mutations were the focus of this particular study.
Summary of Recent BRCA Prevalence Studies
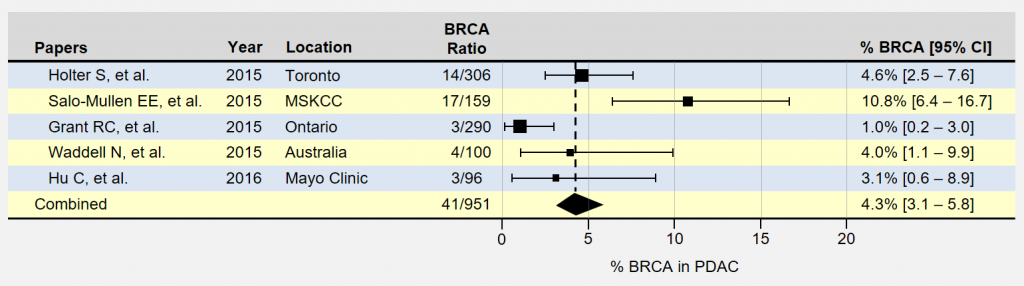
The forest plot below summarizes a few recent papers which include trying to find the approximate percentage of patients with pancreatic cancer who also have inherited BRCA1 and BRCA2 mutations. The position of the rectangles shows the percentage of BRCA1 and BRAC2 patients in a study and the size of the rectangle corresponds to the number of participants. The horizontal lines extending outward represent the level of uncertainty in the measurement. Smaller studies have more uncertainty and longer lines. The last row combines the measurements from all of these listed pancreatic cancer studies into a single measurement. In these five papers the number of those with positive BRCA status is 41 out of a total number with pancreatic cancer of 951 yielding a percentage of 4.3%.
Patient Selection Affect Results
You may have noticed the large differences in BRCA % occurrence in pancreatic cancer found within the papers above. Results can vary significantly between studies for many reasons, such as patient demographics contributing to selection bias. Study locations where BRCA mutations are more common in the population under study can result in much higher rates. For instance, 56% of the patients in the MSKCC study (Memorial Sloan Kettering Cancer Center is located in New York City) were of Ashkenazi-Jewish (A-J) ancestry, which tends to be higher than the general U.S. population and likely contributed to higher prevalence rates. The advantage of looking at the results of multiple studies is that it tends to smooth out the representation of the study populations.
Study Results
The authors of the study in question focused on identifying patients with inherited BRCA1 and BRCA 2 mutations in the context of pancreatic cancer. Their research examined each subject’s ancestry and family health history, finding certain characteristics associated with inheriting a BRCA mutation. As you can see, they identified 14 BRCA patients out of 306 with pancreatic cancer: 4.6%. The researchers are located in Toronto, Canada.
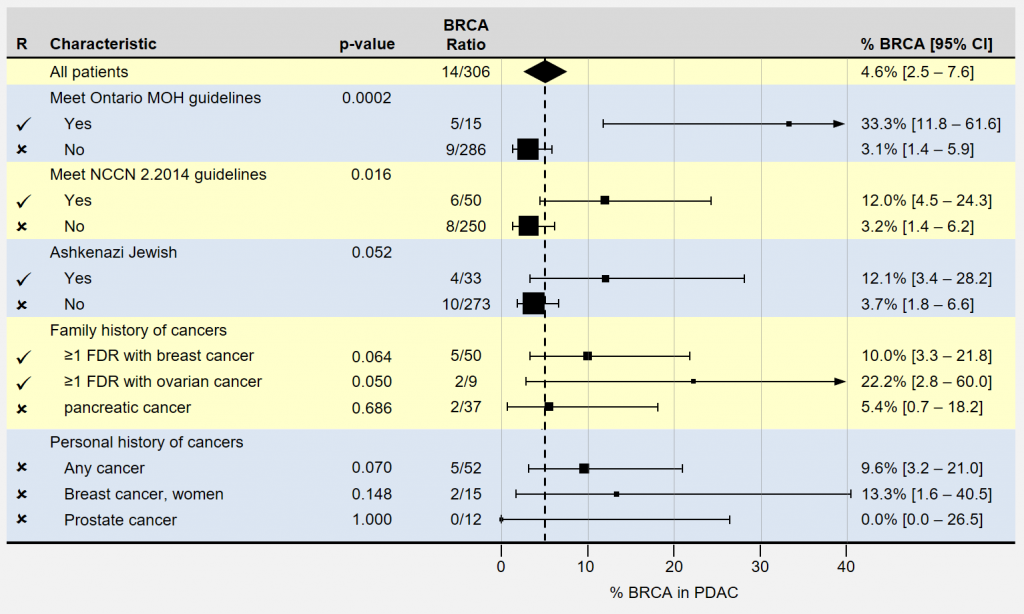
The following forest plot summarizes the characteristics most strongly indicating inherited BRCA1 and BRCA 2 mutations in patients with pancreatic cancer. As before, smaller boxes and longer lines indicate less certainty in the actual prevalence rates. p-values smaller than 0.05 are often used to identify significant results, with smaller values being more significant. The authors recommend genetic screening for patients having characteristics indicated by checkmarks.
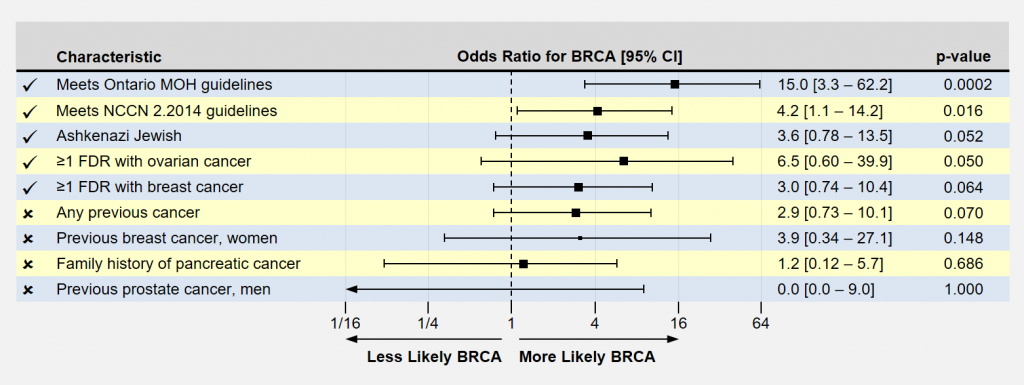
Another way of looking at the results is by computing an odds ratio, presented below. Here, the odds ratio indicates how much MORE likely study participants with the characteristic were to have a BRCA mutation than a reference population. For example, Ashkenazi Jewish patients in this study were 3.6 times more likely to have a BRCA mutation than non-A-J patients.
Significant findings usually have p-values less than 0.05. Longer horizontal lines extending from the box indicate more uncertainty in the result because of smaller sample sizes. In significant findings, you like to see that these lines do not cross 1.0, the ratio of equal likeliness. As one can plainly see, the odds of having a number of these conditions appear to be associated with an increased likelihood of carrying BRCA mutations.
Genetic Screening Recommendations of the Holter, et al. study
The study under question looked at the prevalence, clinical and familial characteristics of pancreatic cancer and recommends genetic screening for the following groups.
- Any Ashkenazi Jewish patients
- Patients with ≥1 first- or second-degree relative with breast cancer
- Patients with ≥1 first- or second-degree relative with ovarian cancer
The authors also suggest that because of the high mortality rate and opportunity for more tailored treatment, all patients with pancreatic cancer might be offered genetic counseling and testing at the time of diagnosis.
Note that the three groups recommended above include any such patients that meet the Ontario’s Ministry of Health (MOH) or NCCN 2.2014 guidelines.
A few of the patient characteristics didn’t quite reach the p<0.05 statistical significance, but were included anyway. Also of note is that patients with “any previous cancer” were not included despite their very similar ratios, confidence intervals, and p-values.
Raising Further Questions
Confusingly, barring an A-J ethnic background, the only groups to be offered genetic testing were identified by familial cancer history, despite their own finding that most BRCA1 and BRCA2 mutation carriers found did NOT have a qualifying family history of cancer.
The authors noted drawbacks to offering genetic testing more widely in regard to pancreatic cancer were related to its low yield and high costs. In an accompanying editorial, these authors also address the family consequences of dealing with BRCA-associated cancers. While valid points, to this blogger, there appears to be no appropriate articulation regarding the potential of the serious up-side for certain patients with pancreatic cancer? For instance, finding a BRCA1 or BRAC2 mutation in a locally advanced or borderline resectable pancreatic cancer patient could dramatically boost their chances at receiving a potentially curative surgical resection. Currently, testing costs for inherited BRCA1 and BRCA2 mutations (as a panel which includes many other genes) can be as low as $250.
The phase 3 clinical trial of the 4-drug FOLFIRINOX regimen (that includes the platinum-based drug, oxaliplatin) for advanced pancreatic cancer saw a 32% overall response rate (ORR), substantially higher than one may have expected (a 2009 phase 3 trial of gemcitabine/oxaliplatin had a 27% ORR). Given the results and considerations of this and other recent studies, this blogger is forced to consider, could some of these responses be due to an outsize effect of oxaliplatin on pancreatic cancer patients in the studied cohort with unidentified germline or somatic BRCA (or other) mutations? And further, would genetic testing of FOLFIRINOX responders with pancreatic cancer be clinically beneficial before a later change to a subsequent treatment that might not include a platinum or other DNA crosslinking agent?
Summary
Prevalence studies like the one in question are finding inherited BRCA1 and BRAC2 mutation at 4-6% of patients with pancreatic cancer. If identified, these potential “Platinum Responders” might better respond to tailored therapy. The effect of discussions along lines as suggested by this paper, given the increasingly lower costs and availability of genetic testing, cause this blogger to wonder if such testing ought not be sought comprehensively for those with pancreatic cancer, testing BRCA genes but also for those select Fanconi Anemia genes whose mutations appear to be related to increased prevalence of pancreatic cancer. Though various sources one is left with an impression that in toto pancreatic cancer “Platinum Responders” may be up to two or three times the number of those with germline BRCA mutations. Time and further research will tell. And finally, the lack of universally agreed upon screening guidelines for pancreatic cancer from authoritative sources are noted to be a limitation for the provider community.
David Dessert
